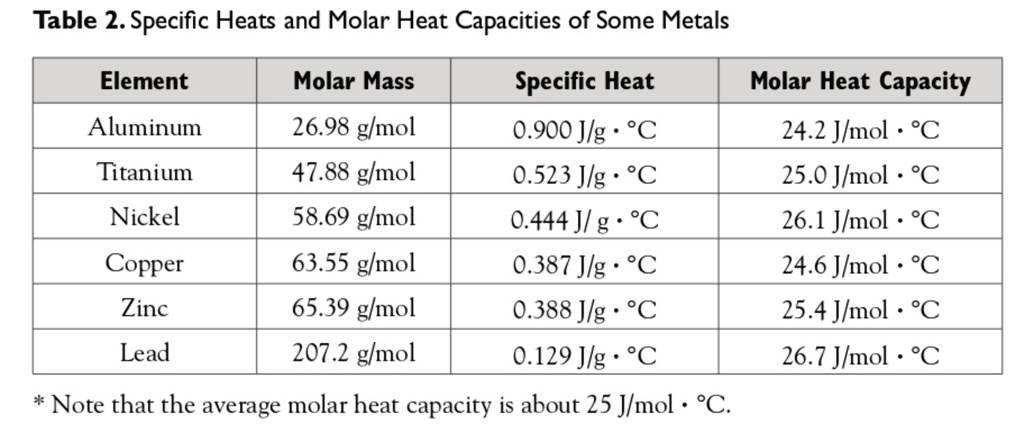
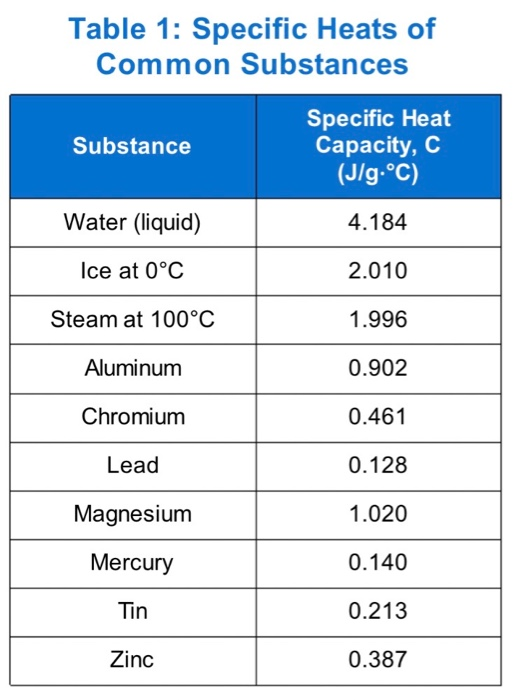
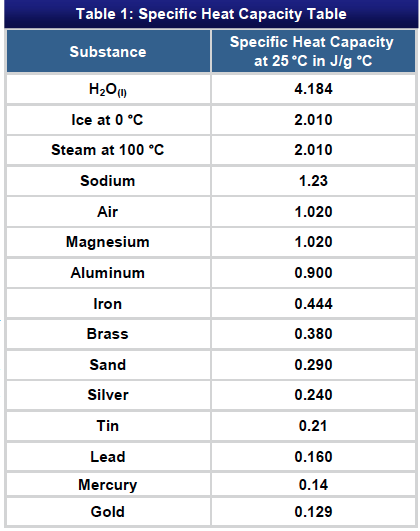
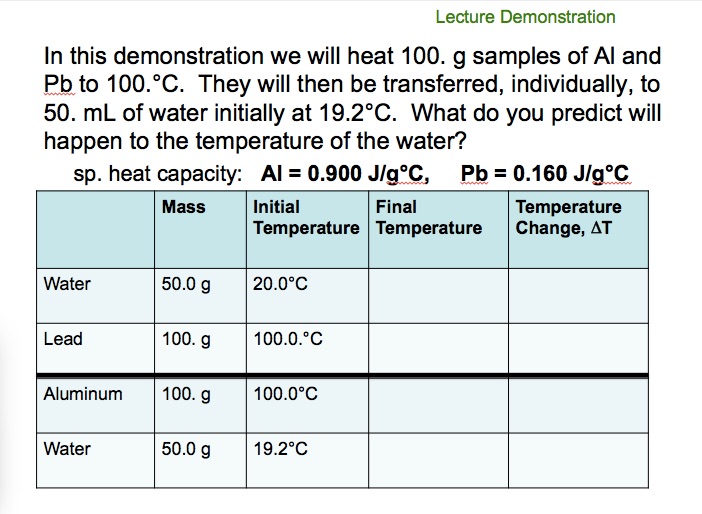
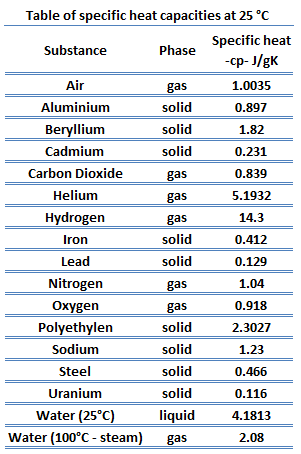
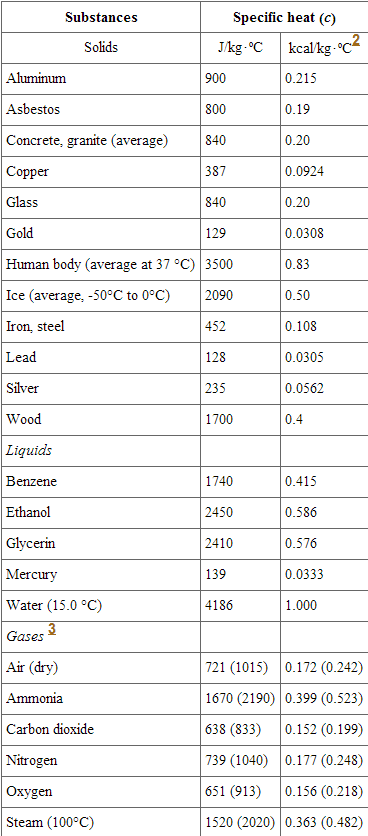
![PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/b0d76592f078aeeb6e66f500b45a1f101c6fe150/6-Table3-1.png)
PDF] A NEW CORRELATION FOR THE SPECIFIC HEAT OF METALS, METAL OXIDES AND METAL FLUORIDES AS A FUNCTION OF TEMPERATURE | Semantic Scholar

PDF) A new correlation for the specific heat of metals, metal oxides and metal fluorides as a function of temperature

LAB: Specific Heat of a Metal. Prelab question: MetalSpecific Heat (J/g ºC) Aluminum0.91 Iron0.46 Lead0.13 Silver0.23 Tin0.21 Titanium0.54 Zinc0.39 A. - ppt download
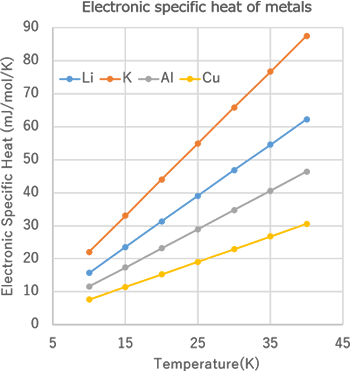
Electronic specific heat analysis of metals - J-OCTA Case Studies | CAE Solutions - JSOL Corporation

PDF) A new correlation for the specific heat of metals, metal oxides and metal fluorides as a function of temperature
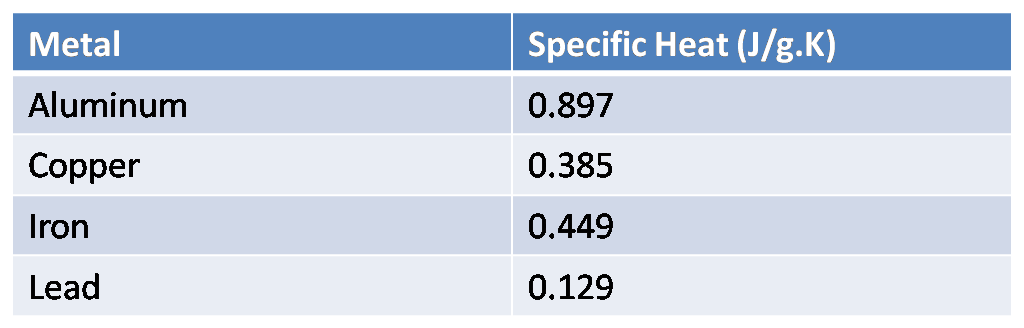
The table below shows the specific heats of several metals. The temperature of a 15-g sample of an unknown metal increases from $20{}^\\circ C$ to $30{}^\\circ C$ when it absorbs 67.5 J
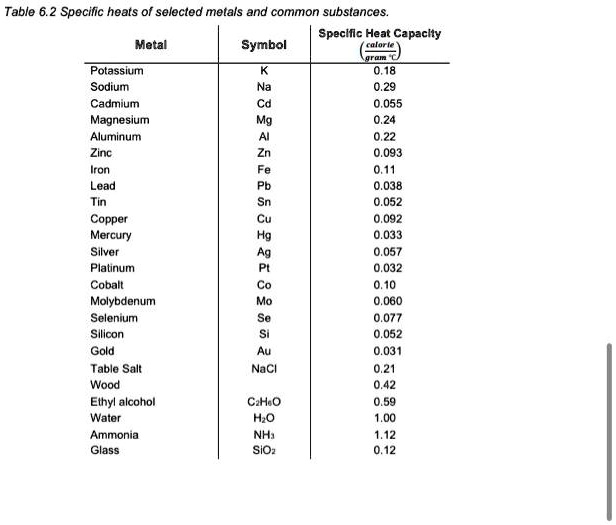
SOLVED: Table 6.2: Specific heats of selected metals and common substances Metal Symbol Specific Heat Capacity Potassium K 0.18 Sodium Na 0.55 Cadmium Cd 0.93 Magnesium Mg 0.11 Aluminum Al 0.038 Zinc

and specific heats of various metals; temperature increments in those... | Download Scientific Diagram
How does heat capacity of copper (or aluminum) change with temperature, when the temperature is in the region of 0°C to 80°C and why? - Quora
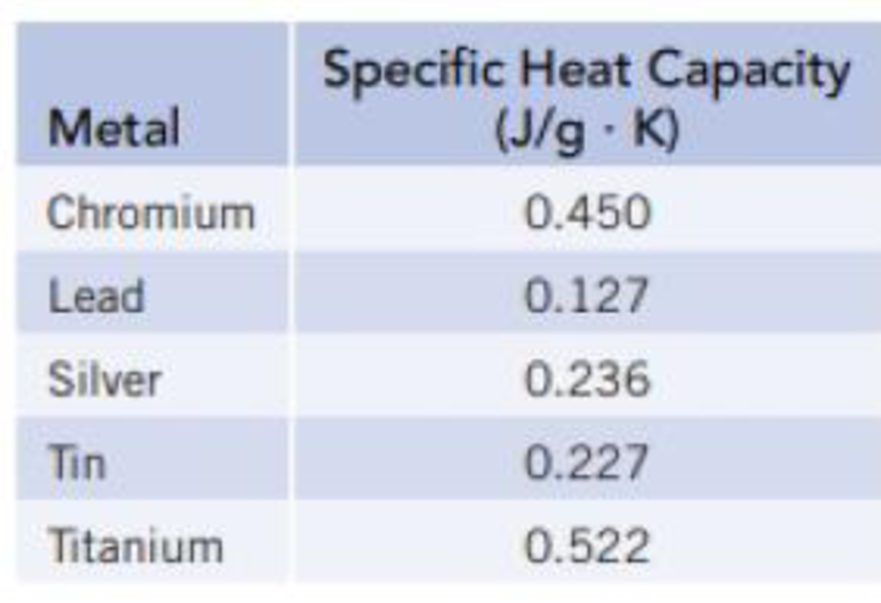
Prepare a graph of specific heat capacities for metals versus their atomic weights. Combine the data in Figure 5.4 and the values in the following table. What is the relationship between specific
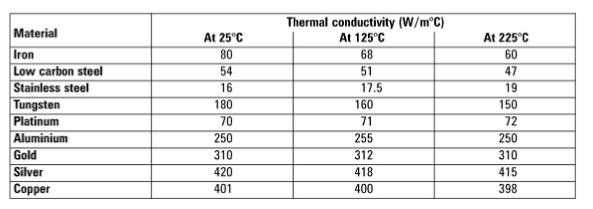
Which metal achieves the highest temperature when adding energy? Aluminum, Copper, or Silver? | CIDER