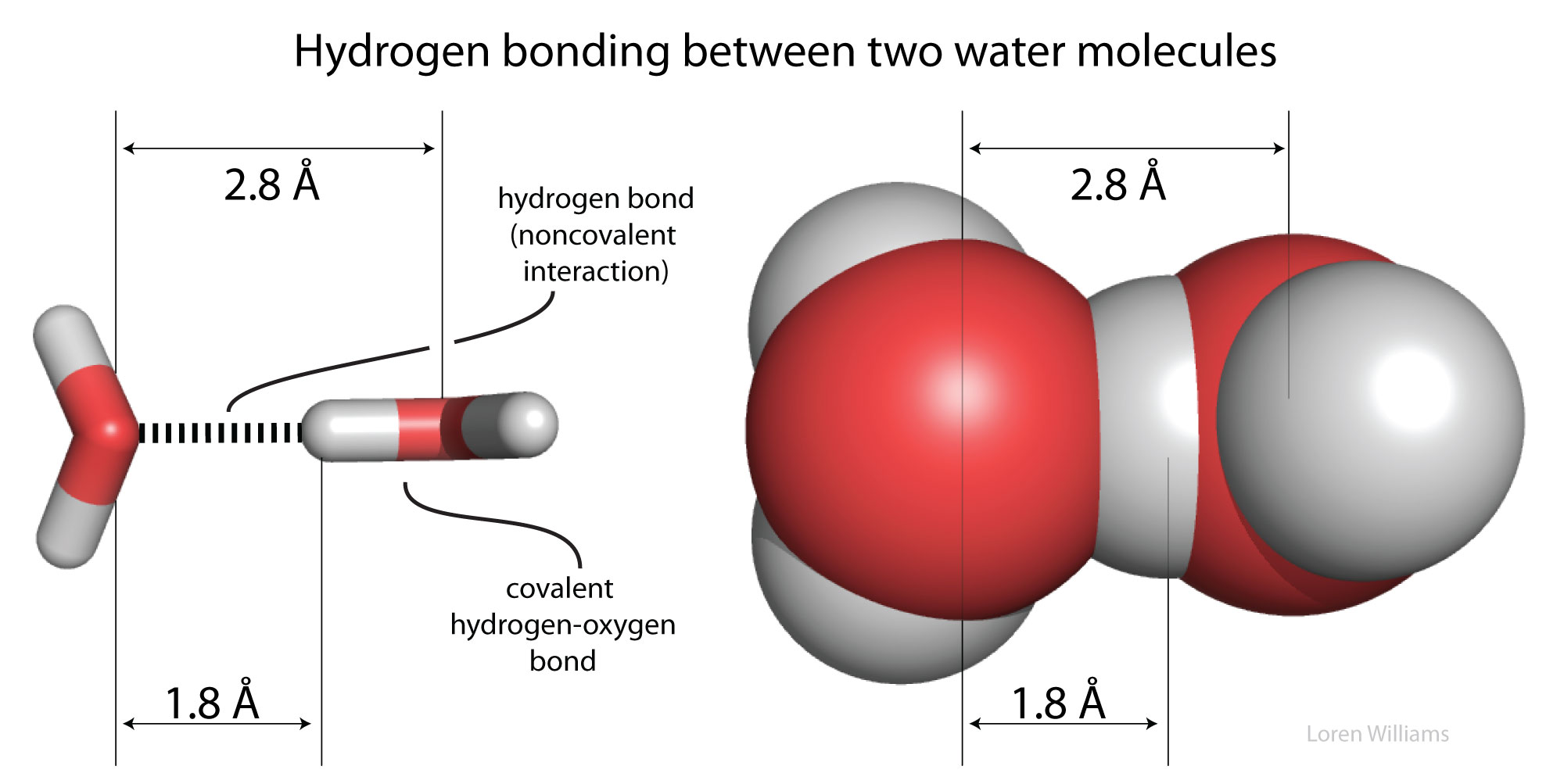
Novel analytical expressions for determining van der Waals interaction between a particle and air–water interface: Unexpected stronger van der Waals force than capillary force - ScienceDirect
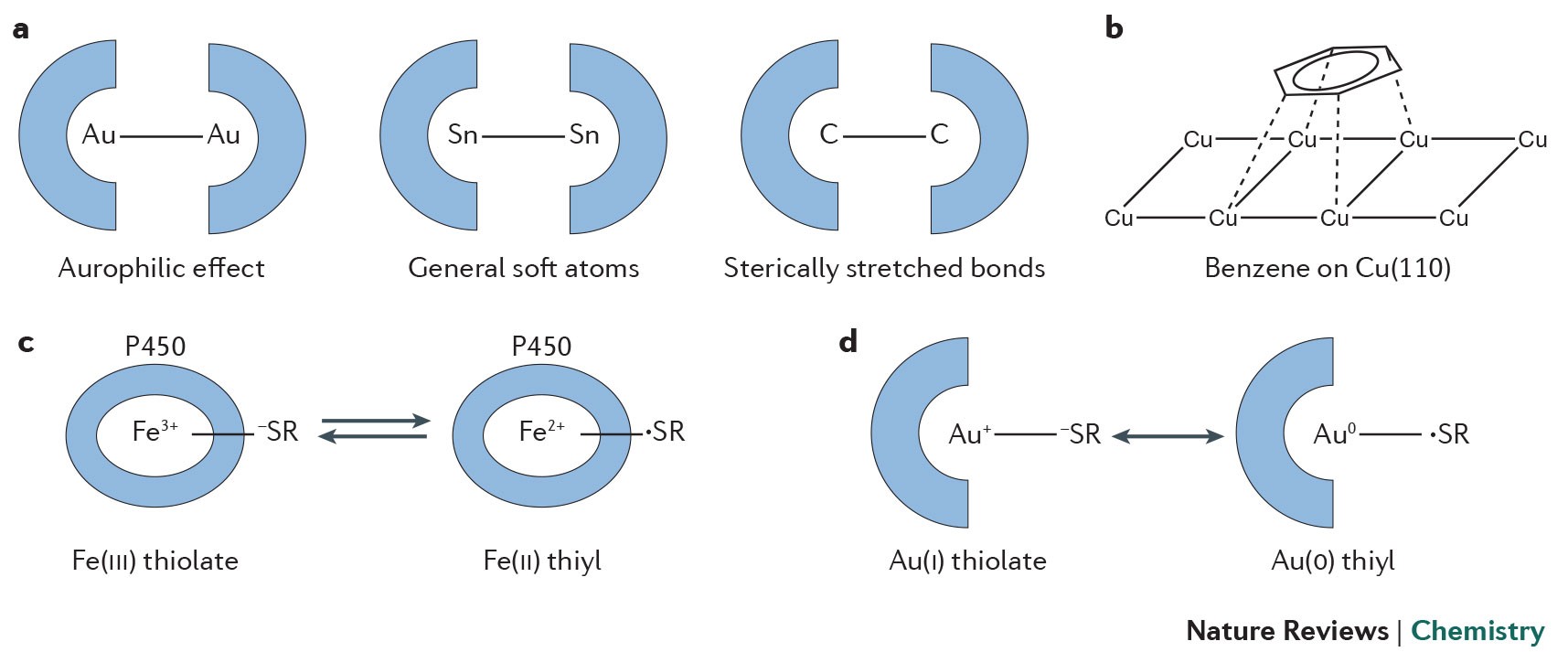
Competition of van der Waals and chemical forces on gold–sulfur surfaces and nanoparticles | Nature Reviews Chemistry

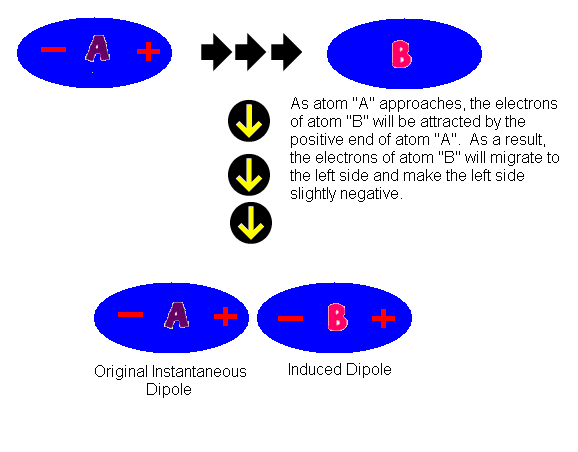
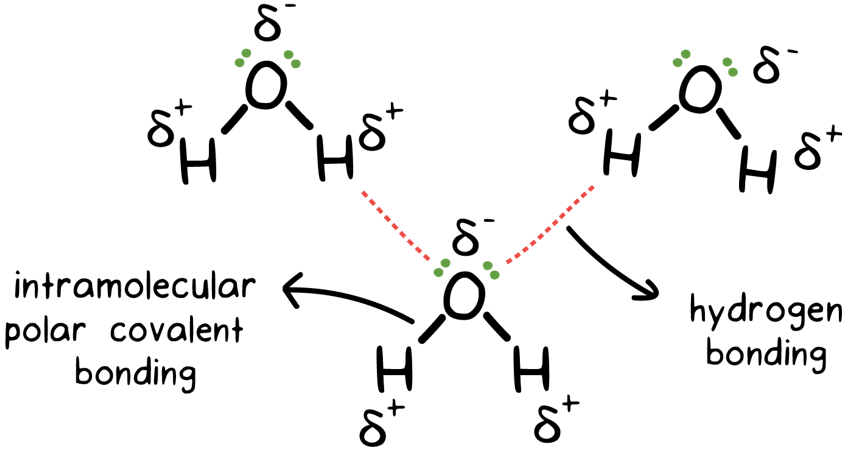
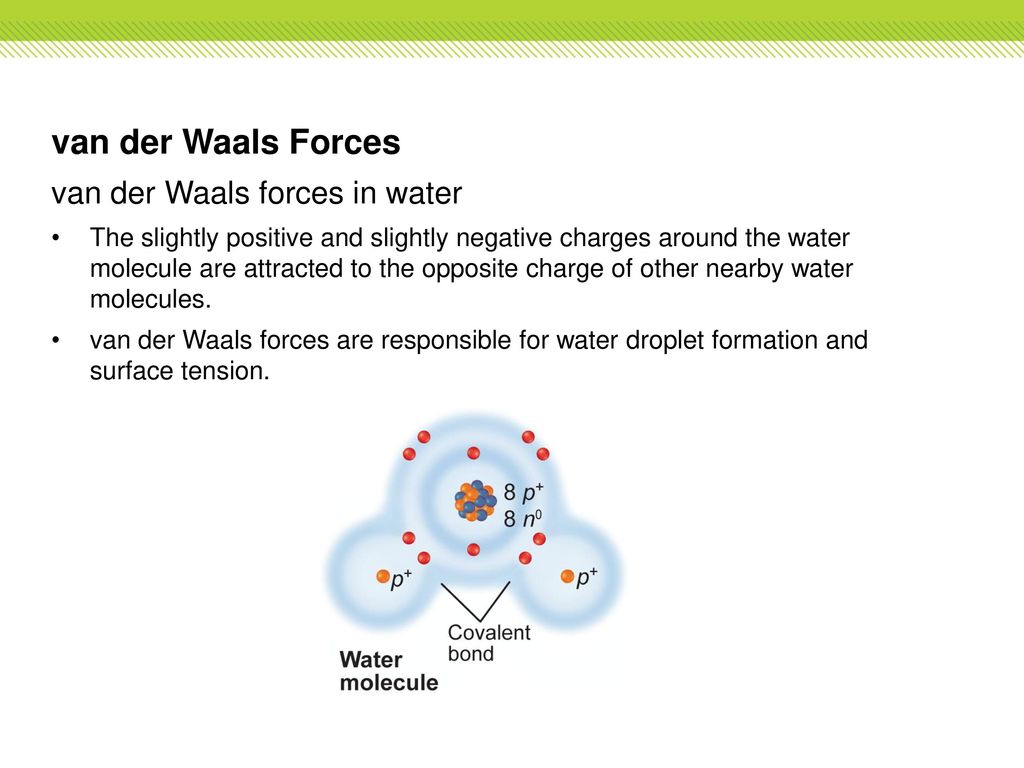
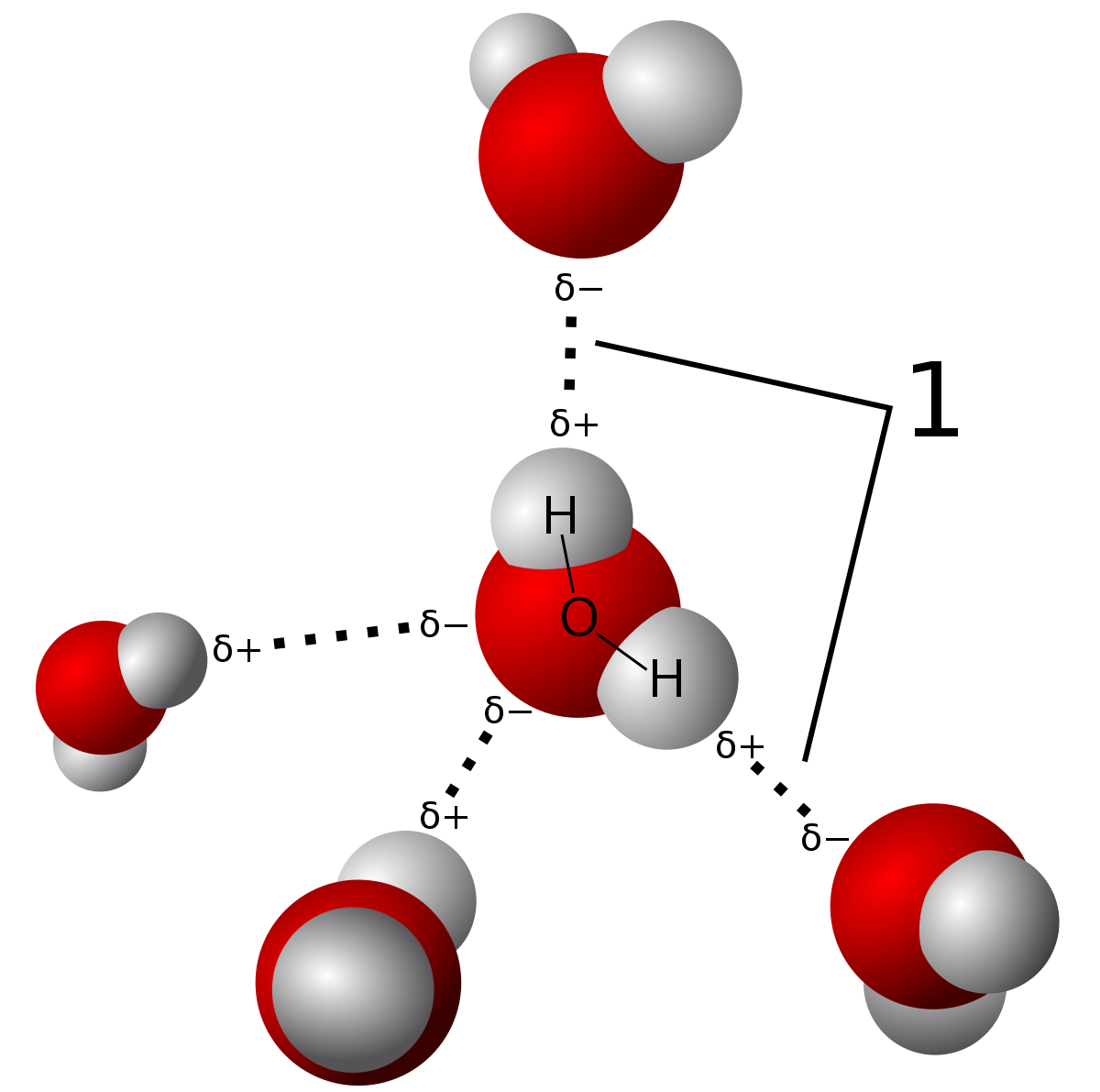
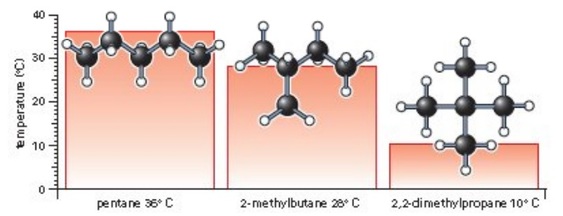
Intermolecular Attractions: Attractions between molecules Van der Waals Forces Dipole interactions Dispersion forces Hydrogen Bonds. - ppt download

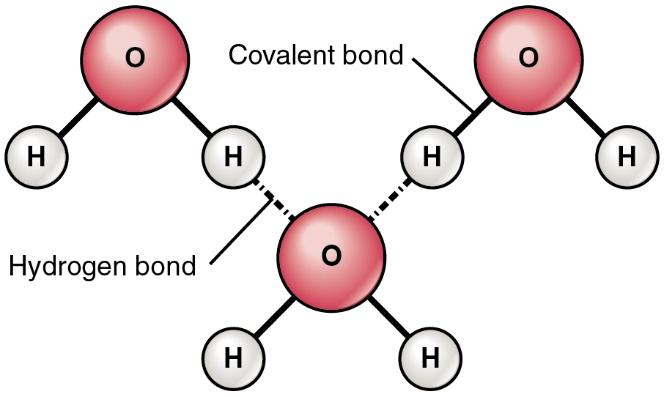
Difference Between Van der Waals and Hydrophobic Interactions | Compare the Difference Between Similar Terms