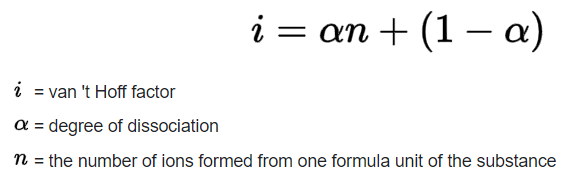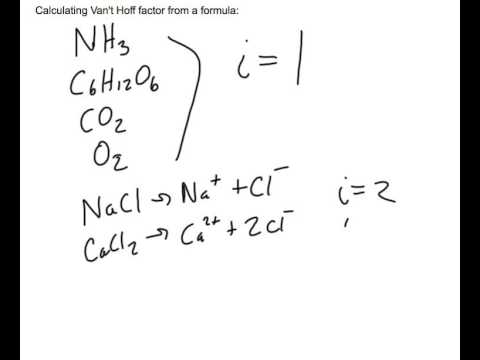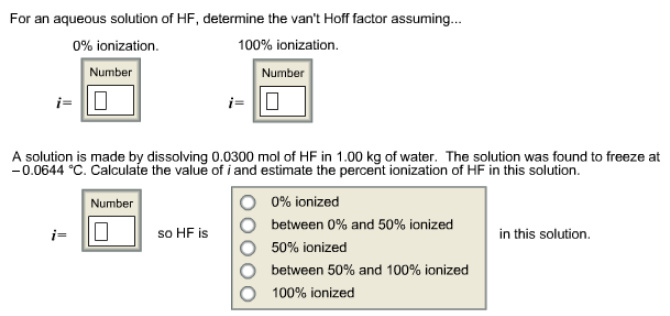
For an aqueous solution of HF, determine the van't Hoff factor assuming - Home Work Help - Learn CBSE Forum
![In which of the following case, van't hoff factor is greater than 3 (A) mathrm{AlCl}_{3} alpha=0.8 (B) mathrm{BaCl}_{2} alpha=0.9 (c) mathrm{NaCl} alpha=0.9 (D) mathrm{K}_{4}left[mathrm{Fe}(mathrm{CN})_{6}right] alpha=0.5 In which of the following case, van't hoff factor is greater than 3 (A) mathrm{AlCl}_{3} alpha=0.8 (B) mathrm{BaCl}_{2} alpha=0.9 (c) mathrm{NaCl} alpha=0.9 (D) mathrm{K}_{4}left[mathrm{Fe}(mathrm{CN})_{6}right] alpha=0.5](https://toppr-doubts-media.s3.amazonaws.com/images/4390395/f7a7e9bd-2451-436d-b371-2afa82a2f7d0.jpg)
In which of the following case, van't hoff factor is greater than 3 (A) mathrm{AlCl}_{3} alpha=0.8 (B) mathrm{BaCl}_{2} alpha=0.9 (c) mathrm{NaCl} alpha=0.9 (D) mathrm{K}_{4}left[mathrm{Fe}(mathrm{CN})_{6}right] alpha=0.5
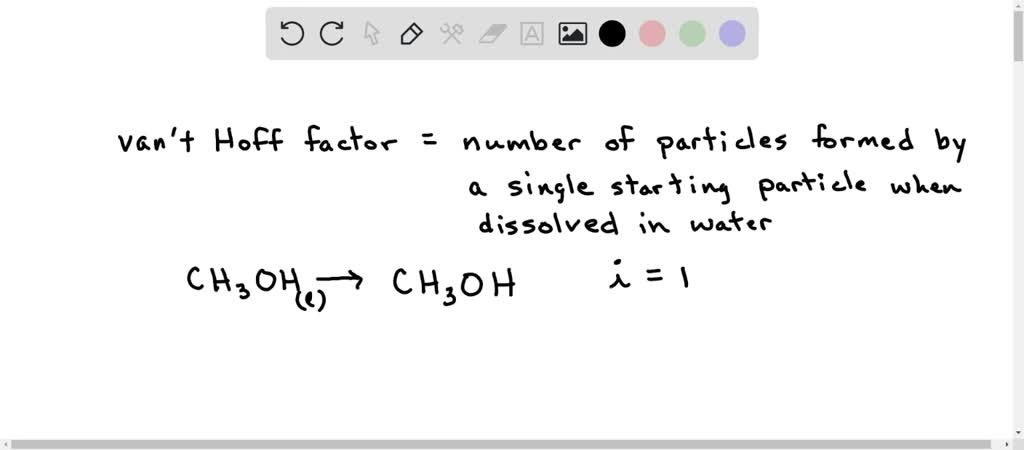
SOLVED: What are the ideal van't Hoff factors for the following compounds, respectively? CH3OH, KOH, HF; CaCl2 A. 2, 1, 3, 2 B. 1, 2, 2, 3 C. 3, 1, 3, 4 D. 0.2, 3, 1, 4 E. 3, 1, 4, 3